The GMC is currently running a consultation on the rules, standards and guidance by which it will, from the end of 2024, regulate physician associates (PAs) and anaesthesia associates (AAs).
Changes to the role of the GMC, and how and who it regulates, are nothing new. GMC archivist Courtney Brucato looks back at some of the milestones in the evolution of medical regulation in the UK.
1878-1956 – regulation of dentists
It was 20 years after the formation of the GMC that the Dentists Act 1878 gave it the additional duty of regulating dentists. The first Dentists Register, published by the GMC in 1879, contained the names of 483 dentists who held a qualification, and 4,800 who claimed to have already been practicing dentistry prior to the passage of the Dentists Act.
The GMC Registrar at the time, Mr William J C Miller, headed up an operation to scrutinise such applications to weed out such persons ‘who can only have been at the time engaged in the practice of dentistry by assisting at the removal of their own milk-teeth.’
A standardised dental curriculum was adopted by the GMC in 1879 and communicated to the relevant licencing bodies [image below]. The GMC’s Dental Committee also recommended that examinations should ‘be of a practical character, and should include actual operations and the preparation of specimens of Mechanical Dentistry.’
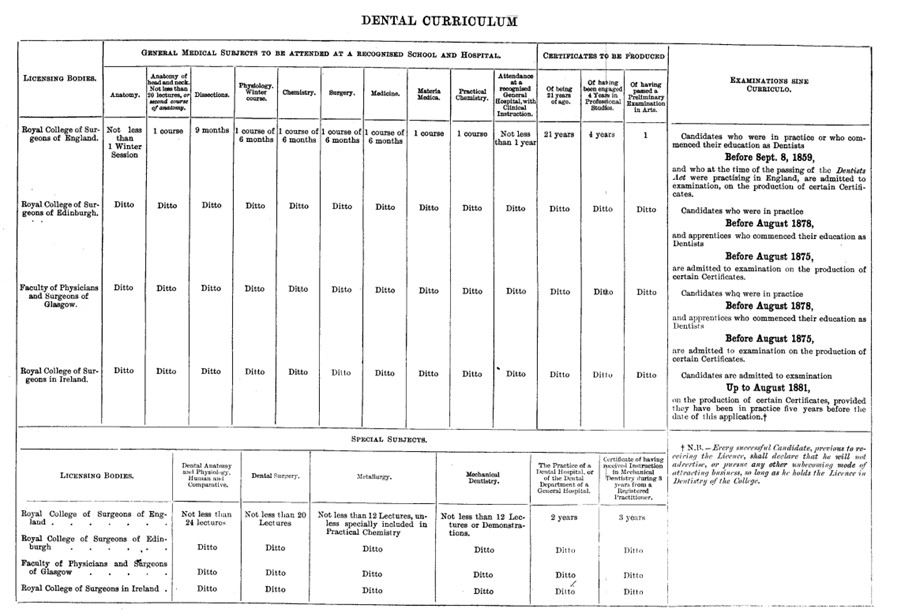
In 1921, the registration function of maintaining the Dental Register was transferred to the newly formed Dental Board of the United Kingdom. However, The GMC retained powers relating to dentistry’s educational standards and disciplinary functions.
It wasn’t until the passage of the Dentists Act 1956 that the GMC transferred all remaining functions to the newly formed General Dental Council, which exists to this day.
1864-1963 – The British Pharmacopoeia
Pharmacopoeias – volumes that describe ingredients and preparations of medicines – date back to ancient times. The first pharmacopoeia in the UK that set a binding standard was the 1618 Pharmacopoeia Londinensis, created jointly between the College of Physicians and London apothecaries. This was eventually superseded by the publication, by the GMC in 1864, of the British Pharmacopoeia. It combined the London, Edinburgh and Dublin pharmacopoeias.
Publishing the British Pharmacopoeia was one of the original functions of the GMC, as it was included in the Medical Act 1858 which first established the GMC as the country’s medical regulator. The preparation of the first edition, in 1864, was undertaken by members of the GMC’s Pharmacopoeia Committee, as well as with external experts they consulted.

The GMC’s Pharmacopoeia was divided into two parts with an appendix. Part I consisted of ‘Materia Medica’ (medicinal substances) and Part II the preparations of compounds. The appendix included ‘articles employed in the preparation of medicines’. [Image below extract from appendix]

By 1928, the preparation of the Pharmacopoeia made such extensive use of outside technical expertise that a Pharmacopoeia Commission was established, with a permanent secretary and officers employed by the GMC. The Commission would prepare Pharmacopoeia editions, and these would be approved by the GMC Council and published.
The final edition of the Pharmacopoeia was published by the GMC was in 1968, 104 years after the first edition and 110 years since the GMC was first tasked with the statutory responsibility under the Medical Act of 1858.
The Medicines Act 1968 formally established the British Pharmacopoeia Commission, now The British Pharmacopoeia, which took on responsibility for future British Pharmacopoeias.
1972-1978 – The Merrison Committee and Health Procedures
In 1972, an Inquiry was established by Sir Keith Joseph to:
Consider what changes need to be made in the existing provisions for the regulation of the medical profession; what functions should be assigned to the body charged with the responsibility for its regulation; and how that body should be constituted to enable it to discharge its functions most effectively; and to make recommendations.
The inquiry was chaired by Professor Alexander W. Merrison, F.R.S. The Merrison Committee’s recommendations were incorporated within the Medical Act 1978, and extended the GMC’s functions in the areas of standards and medical education, as well as establishing a new Health Committee to consider concerns about doctors suffering from ill health.

The GMC sought health procedures because previously it could do nothing with cases of sick doctors other than act through disciplinary machinery. Imposing a suspension or conditions on a doctor’s registration, when necessary to protect the public, was intended to compliment local procedures.
Health assessments for doctors experiencing physical or mental ill health still form part of our procedures today. You can read here an account of a doctor’s experience with the GMC health process and recovering from their illness.
2010 – Specialist registration and merger with PMETB
In April 2010, the Postgraduate Medical Education and Training Board (PMETB) formally merged with the GMC. From this point on, there was a single organisation responsible for all stages of medical education in the UK.

PMETB officially took up its remit in 2005, but it actually began work two years earlier after the passage of the General and Specialist Medical Practice (Education and Qualifications) Order in 2003.
It issued curricula standards, established an assessment system for postgraduate medical training, and approved new equivalence routes to the GMC’s Specialist and GP Registers.
The merger between the GMC and PMETB came following the publication of the final Tooke Report – Aspiring to excellence – in 2008. One of the recommendations was for undergraduate and postgraduate training to be regulated by the GMC. The recommendation was welcomed by both the GMC and PMETB.
2012 – Introduction of revalidation
We recently passed the 10-year anniversary of the implementation of revalidation, the process by which doctors holding a licence to practise demonstrate that their knowledge and skills are up to date and that they remain fit to practise.
The seeds from which revalidation as we know it today date from at least 1973 when the Board of Science and Education of the British Medical Association set up a series of exploratory meetings. It was from these meetings that the Committee of Inquiry into Competence to Practise evolved. This Committee included representatives from all medical royal colleges and their faculties, the Joint Consultants Committee and the BMA.
The Committee was chaired by Sir Anthony Alment and the terms of reference were:
‘To review the present methods of ensuring the maintenance of standards of continuing competence to practise and of the clinical care of patients, and to make recommendations.’
The committee’s report was published in 1976 but it was largely overshadowed by the work of the Merrison Committee and subsequent legislative changes that resulted from it.
But, in the years that followed, serious failings such as the Bristol Royal Infirmary heart scandal and the Harold Shipman case propelled the medical profession to again consider the need for revalidation.
Revalidation was formally launched on 3 December 2012, making the UK the first country in the world to introduce such a process across its whole healthcare system, covering GPs, hospital doctors, locums and those working in the independent sector.
Professor Sir Peter Rubin, then Chair of the GMC, was the first doctor to revalidate later that month. He remarked:
‘I am delighted to be the first doctor in the UK to revalidate. This is the biggest change to medical regulation since the GMC was established in 1858 and change always brings some uncertainty to those it affects.
‘However, to my medical colleagues I’d say that in this age of transparency our patients will expect nothing less. I’ve had a number of patient and colleague feedbacks over the last few years and they’ve been helpful – partly in reaffirming all the things I do well and also in identifying what I can do better; none of us is perfect. For the vast majority of doctors, revalidation will be about improving still further their high standards of practice.’
2024 – Bringing PAs and AAs into regulation
The GMC’s role and ways of working have been evolving ever since it was formed in 1858. In part it’s a response to the changing nature of medicine and healthcare, and in part it’s a commitment to continuous improvement and to working with our stakeholders.
The next major change will see the GMC become, once again, a multi-disciplinary regulator. By the end of 2024 the GMC will regulate physician associates and anaesthesia associates, as well as doctors. A consultation on the rules for this underway now, and runs until midnight on Monday 20 May. For more information and to get involved click here.

